Overview
Gene therapy aims to fix genetic defects causing diseases by introducing functional copies of genes into a patient’s cells to restore their normal activity.
Over the past decade, gene therapies have begun to demonstrate their potential through notable clinical successes and several regulatory approvals.
Lentiviral vectors offer the advantage of long-term expression of therapeutic genes and are particularly adapted to multiplying cells and growing organs, where non-integrative approaches, such as adeno-associated vectors (AAVs), lead to suboptimal results. Lentiviral vectors, however, can generate immune responses, which limits their use.
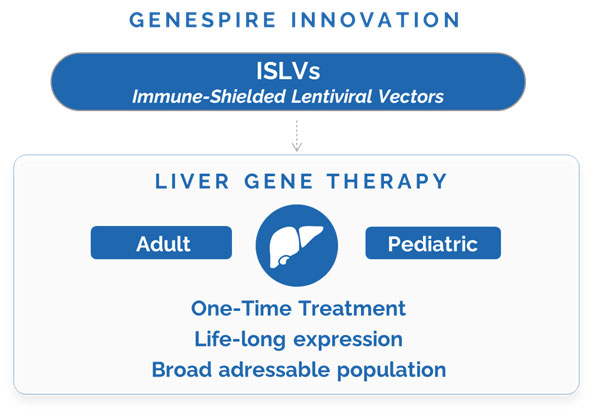
Genespire has generated a novel proprietary class of lentiviral vectors, the Immune Shielded Lentiviral Vectors (ISLVs), which are masked from the patient’s immune system. They are designed to be injected directly into the patient’s peripheral blood, where they can travel to the liver and insert the therapeutic gene into the DNA of the patient’s cells.
This integration allows the therapeutic gene to be maintained through cell division, ensuring its stable expression in a growing liver. Therefore, a life-long therapeutic effect of ISLVs is expected, even when patients are treated at a young age.
This innovative platform, with application in many genetic diseases, promises to extend the reach of in vivo gene therapy, offering a one-time, off-the-shelf treatment for both adult and pediatric patients.